A Research Consent Form is a crucial document that ensures participants fully understand the nature of a study before agreeing to take part. Informed consent safeguards both participants and researchers, offering transparency on risks, benefits, and expectations. This guide will walk you through how to create an effective consent form, complete with Consent Form and Research Informed Consent Form. Whether you’re conducting medical trials, social research, or surveys, this detailed guide will ensure your forms meet ethical standards and provide clear information to participants.
What is Research Consent Form?
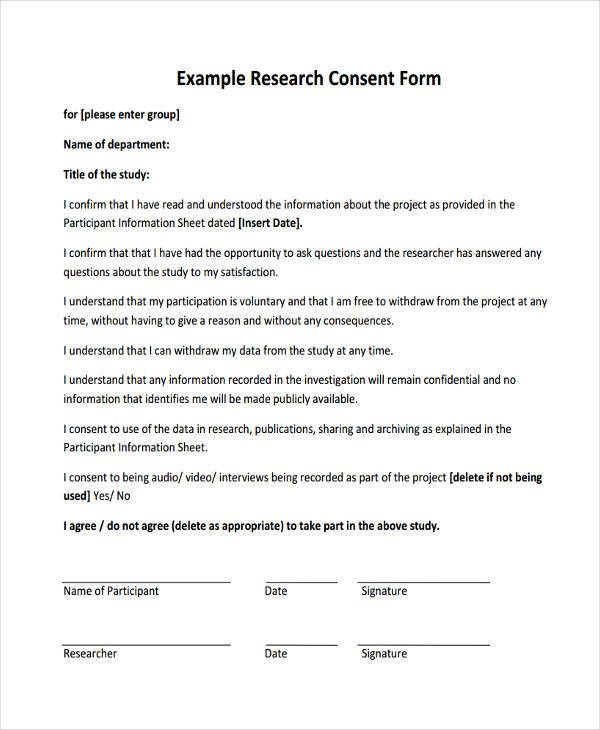
A Research Consent Form is a document that outlines the key details of a study to participants, ensuring they fully understand what they are agreeing to. It explains the study’s purpose, procedures, potential risks, and benefits. The form is essential for protecting participants’ rights, allowing them to make an informed decision about their participation. Researchers must ensure the form is clear, easy to read, and covers all necessary information. Informed consent is fundamental in ethical research practices.
Research Consent Form Format
Research Study Title:
Principal Investigator:
- Name: ______________________________
- Contact Information: __________________
Purpose of the Study
- Briefly explain the research purpose and why the participant is being asked to participate.
Procedures
- Detailed description of what the participant will do during the study.
- Time commitment required: __________________
- Location: _______________________________
Risks and Benefits
- Clearly outline any potential risks, discomforts, and benefits to the participant.
Confidentiality
- Explanation of how participant’s information will be kept confidential.
Voluntary Participation
- Emphasize that participation is voluntary and that participants can withdraw at any time without penalty.
Informed Consent
- I, ____________________, have read and understood the details of the study. I voluntarily agree to participate.
- Signature of Participant: ___________________
- Date: ___________________
Investigator’s Signature
- Signature: ___________________
- Date: ___________________
Sample Consent Form for Research

A Sample Consent Form for Research provides a clear example of how to outline participant rights, study details, and potential risks. This form can serve as a template for creating your own, ensuring compliance with ethical standards. It can also be adapted for Field Trip Consent Form use.
Consent Form for Research PDF

Downloadable and printable, a Consent Form for Research PDF offers convenience for researchers and participants alike. Ensure all necessary details, such as study objectives and participant confidentiality, are included. This format is also useful for Photo Consent Form purposes.
Short Consent Form for Research

A Short Consent Form for Research is a concise version of a typical consent form, offering the most critical information in a simplified format. It’s ideal for studies requiring quick approval and is easily adaptable for Child Travel Consent Form situations.
Consent Form for Research Interview

A Consent Form for Research Interview secures participant agreement to be recorded or transcribed during interviews. It outlines how their data will be used. This form can also apply to scenarios involving a Minor Travel Consent Form, ensuring legal compliance.
Browse More Research Consent Forms
Psychology Research Consent Form
Medical Research Consent Form
Free Research Consent Form
Research Participant Consent Form
Research Informed Consent Form
Generic Research Consent Form
Research Interview Consent Form
Research Consent Form Example
Simple Research Consent Form
How do you write a consent form for research?
Writing a Research Consent Form involves outlining the study details, participant rights, and ensuring informed consent is obtained. Key steps include:
1. Study Purpose: Clearly explain the purpose of the study to the participant.
2. Procedures: Describe the procedures the participant will undergo during the study.
3. Risks and Benefits: Outline potential risks and benefits for the participant.
4. Confidentiality: Ensure participant information will be kept confidential and secure.
5. Voluntary Participation: State that participation is voluntary and the participant can withdraw at any time. You also browse our Model Consent Form
What are the components of a consent form?
A well-structured Research Consent Form must include four essential components to be effective. These components are:
1. Study Information: A clear explanation of the research, including its purpose and scope.
2. Procedures: Step-by-step details on what the participant will do.
3. Confidentiality: Assurance of how personal data will be protected.
4. Voluntary Participation: A section explaining the participant’s right to withdraw.
5. Signatures: Spaces for the participant and researcher to sign, ensuring formal agreement. You also browse our Financial Consent Form
What are the pillars of consent?
The three pillars of consent in research ensure that participation is ethical and valid. These pillars are:
1. Informed Consent: Participants are fully informed about the study.
2. Voluntary Participation: Participation is not coerced or forced in any way.
3. Capacity to Consent: Participants are mentally and legally capable of providing consent, such as through a Passport Consent Form for travel studies.
4. Parental Involvement: When minors are involved, ensure the Parent Consent Form is signed.
5. Business and Legal Boundaries: Special cases may require a Check Consent Form to align with organizational or legal procedures.
What is consent to participate in a research study?
Consent to participate in a research study is the formal agreement from participants to take part, after being fully informed. Steps include:
1. Understanding Study Purpose: Participants need to understand why the research is conducted.
2. Voluntary Agreement: Consent must be freely given without pressure.
3. Data Usage Disclosure: How the data will be used must be clear to the participant.
4. Risk Explanation: Participants should know any risks or potential harm involved.
5. Withdrawal Option: Participants must be informed they can withdraw anytime, similar to opting out of a Business Consent Form.
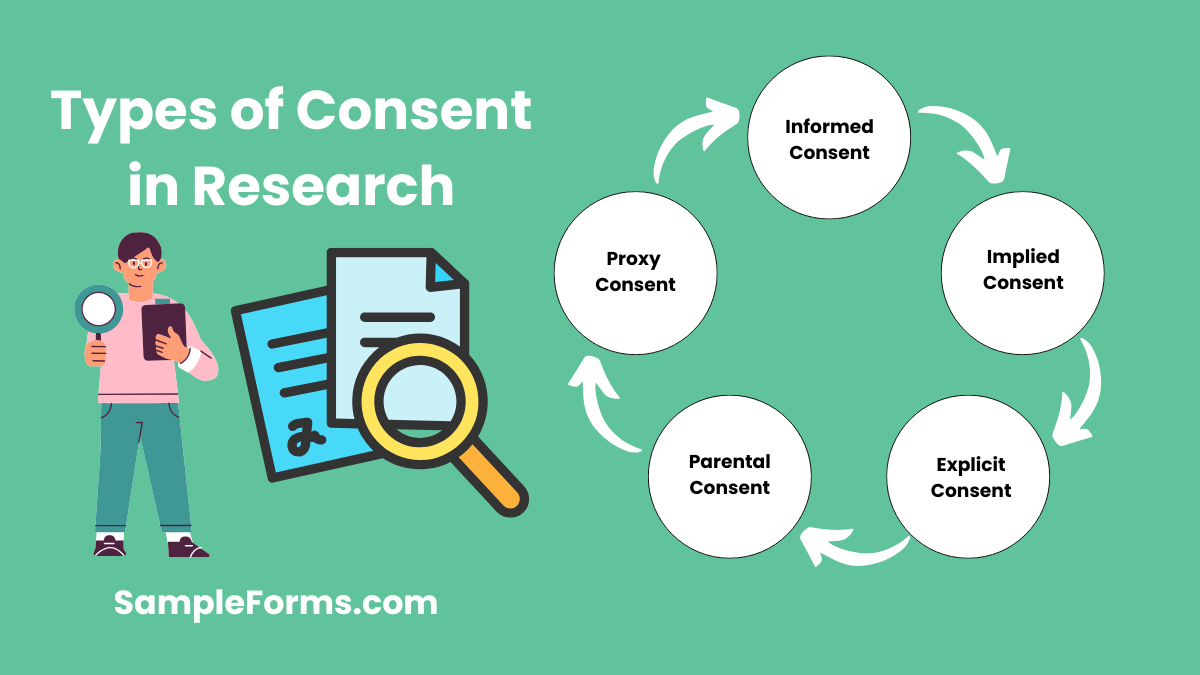
What are the types of consent in research?
Understanding the four types of consent is crucial for researchers when obtaining participant approval in a study. These types include:
1. Informed Consent: Participants receive complete information before agreeing to take part.
2. Implied Consent: Consent is assumed based on actions or participation.
3. Explicit Consent: Written or verbal agreement to participate.
4. Parental Consent: Required when minors are involved, often through a Parental Consent Form.
5. Proxy Consent: Given by a third party if the participant is unable to consent.
Can I make my own consent form?
Yes, you can create your own Client Consent Form by including essential elements like study purpose, procedures, risks, and voluntary participation. Ensure it follows ethical guidelines and standards for proper participant consent.
What does written consent look like?
Written consent includes a signed document where the participant agrees to take part in the research, understanding the purpose, risks, and confidentiality, such as in an Interview Consent Form for recorded conversations.
How do you write a brief consent form?
A brief consent form clearly outlines the study’s purpose, potential risks, and participant rights while maintaining simplicity. It should still ensure understanding, similar to a Surgical Consent Form for medical procedures.
What is a consent template?
A consent template is a pre-structured document that includes sections for purpose, risks, procedures, and participant rights, which can be adapted for various uses, such as a Counseling Consent Form for therapy sessions.
How to write a research consent form?
To write a Research Consent Form, detail the study’s purpose, risks, and confidentiality. Ensure participants know their rights and that participation is voluntary, similar to preparing a Child Medical Consent Form for minors.
What 3 things are needed for consent?
For consent to be valid, it must involve informed understanding, voluntary participation, and the competence of the participant to agree. This structure applies to various forms, including a Survey Consent Form.
What is not considered consent?
Consent is not valid if obtained through coercion, manipulation, or misunderstanding. An example is when a Questionnaire Consent Form does not fully inform the participant about the study’s purpose and risks.
What is an example of consent for a survey?
An example of survey consent would be: “I voluntarily agree to participate, fully understanding the study’s purpose and confidentiality.” This format can be adapted for forms like a Privacy Consent Form for data protection.
What are the research consent questions?
Research consent questions typically ask about the study’s purpose, risks, confidentiality, and withdrawal rights. This type of form could resemble a Landlord Consent Form in terms of outlining specific agreements.
How do you write a short consent form?
A short consent form includes the study’s purpose, confidentiality, risks, and a statement of voluntary participation. It should be concise but clear, similar to the format of a Dental Consent Form in healthcare settings.
In conclusion, the Research Consent Form is an indispensable part of any ethical study. Whether you’re drafting a Vaccine Consent Form, or any other research-related document, proper formatting and content are essential. Clear, concise, and legally compliant forms ensure participant understanding and protect against potential liabilities. By using this guide and examples provided, you can create effective Research Consent Forms, Samples, and Letters tailored to your research needs.